"Written on the front" The people s daily published an article questioning
On April 17, the CCTV on Changzhou School of foreign languages by adjacent chemical plant pollution caused widespread concern about reports of the site. CCTV reported that since the beginning of the end of 2015, foreign language schools in Changzhou has 641 students were taken to hospital for checks. There are 493 people, eczema and dermatitis, bronchitis, abnormal blood parameters, leukopenia and other abnormal symptoms, also was found to have Lymphoma, leukaemia and other malignant diseases.
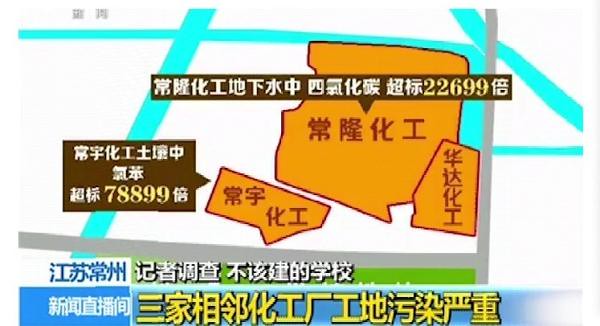
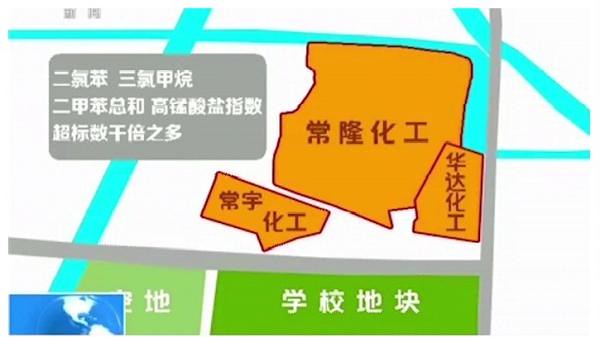
CCTV reported that school is next to the site of three adjacent chemical plant, near land pollution, excessive. According to a project environmental impact: the site soil and groundwater with chloro-organic pollutants such as carbon Tetrafluoride, naphthalene and INDENE and pyrene polycyclic aromatic hydrocarbons and metals such as mercury, lead, cadmium and other heavy metals in common over serious.
Among them, Chang Yu chemical in groundwater, and p-dichlorobenzene 94,799 times times exceeded; Chang Yu chemicals in soil, excessive chlorobenzene 78,899 times; changlong chemicals in groundwater, excessive carbon tetrachloride 22,699 times.
Dichlorobenzene, chloroform and xylene permanganate index exceeds the sum of thousands of times more then just how large these compounds for human damage, the Government has banned the use of these pollutants? Surging News (www.thepaper.CN) through access to authoritative sites at home and abroad, and mentioned in the news nature of several pollutants, harmful.
P-dichlorobenzene
Properties Overview
Colorless liquid, boiling point of 132.2. During the first world war, mainly used in military explosives required for the production of picric acid. Between 1940 and 1960, extensively used to produce DDT (DDT) pesticide. After 1960, DDT has been replaced by the high efficiency and low residual pesticides, while the demand is declining. Many resin and solvent used primarily ethyl cellulose, produces a variety of other benzene intermediates, such as nitro-chlorobenzene.
Use
Dyes, pharmaceutical industry for the manufacture of phenol and p-nitrochlorobenzene, aniline, Nitro-phenol and organic intermediates. The rubber industry for the manufacture of rubber chemicals. Industry for the manufacture of DDT pesticides, paint and coatings used in the manufacture of paint. Light industry for the manufacture of cleaning and quick-drying ink. Chemical production is used as a solvent and heat transfer medium. Used as a chemical reagent in analytical chemistry.
Risk
Restraint and anesthesia effect on the central nervous system; irritating to skin and mucous membranes.
Performance:
Acute toxicity: exposure to high concentrations can cause symptoms of anesthesia, and even coma. From the scene, after active treatment, faster recovery, but still within a few days of headache, dizziness, weakness, loss of appetite and other symptoms. Liquid with slight irritation on the skin, but repeated exposure, redness or slight superficial necrosis.
Chronic poisoning: common eye pain, tears, conjunctival hyperemia; early headaches, insomnia, memory loss and other symptoms of neurasthenia; severe toxic hepatitis, kidney damage can occur.
Environmental hazard: serious harm to the environment, water, soil and air pollution.
Combustion hazard: this product is flammable, irritant.
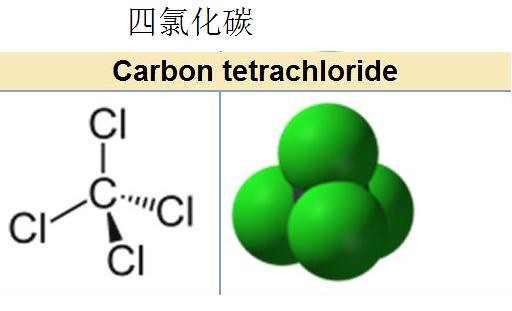
Carbon tetrachloride
Properties Overview
A colorless toxic liquid, dissolve fats, paints and other materials, volatile liquid, with a slightly sweet smell of chloroform. Molecular weight 153.84, density 1.595g/cm ³ at normal temperature and pressure (20 ℃), the boiling point of 76.8, vapor pressure of 15.26kPa (25 ℃), vapor density 5.3g/L. Carbon tetrachloride and water do not mix, but with alcohol, ether, chloroform and ether is miscible.
Carbon tetrachloride is a colorless clear fluid liquid, sometimes contain impurities on the industry is a yellowish, aromatic odor, volatile. Density (20 ℃) 1.595,-22.8 grams/cubic centimeter, melting point, boiling point ℃ 76-77. Carbon tetrachloride steam about 5 times heavier than air and does not burn. Carbon tetrachloride vapors of toxic, it's lower than chloroform anesthesia, but high toxicity. Inhaled 2-4 ml can kill people.
Solubility of carbon tetrachloride in water is very small, light and moisture and that is gradually decomposed into hydrochloric acid. Soluble in various organic solvents, alcohol, ether, chloroform, benzene, and mixed. For fats, oils, and a variety of organic compounds is an excellent solvent.
It is not flammable, served as a fire extinguishing agent, but because it can react with water at above 500 degrees Celsius to produce carbon dioxide and toxic phosgene, chlorine and hydrochloric acid, and it will accelerate the decomposition of the ozone layer, so it is deactivated. Now used to clean carbon tetrachloride most of them have been replaced by trichloroethylene.
Uses of carbon tetrachloride is strictly limited by country, only used for feedstock uses non-ozone-depleting substances and special purposes, is not commonly used as extractant.
Allow access to quota: 25mg/m ³, China; United States 0.005mg/L
Risk
Carbon tetrachloride is a typical liver toxins, but can affect the exposure concentration and frequency of its site of action and toxicity. At high concentrations, starting with central nervous system involvement, and involving the liver, kidney; while the long-term exposure to lower concentration is mainly hepatic and renal involvement. Alcohol can promote the absorption of carbon tetrachloride, aggravate symptoms of poisoning. In addition, carbon tetrachloride may increase myocardial sensitivity to adrenaline, causing serious arrhythmias.
Great difference in people's individual susceptibility to carbon tetrachloride, there have been reports of oral 3~5ml poisoning, 29.5ml to death. Poisoning can occur in 160~200mg/M3 concentrations. But there are also concentrations in 1~2G/M3 contact 30min under mild poisoning. Currently considered CTC no teratogenic and mutagenic effects, but has toxicity. According to information on IARCl972 and in 1979, long term effects of carbon tetrachloride can cause liver cancer in rodents, are classified as "carcinogenic to humans" a class of chemicals.
Risk
1. acute toxicity
(1) incubation period: 1-3 days, there are as short as a few minutes. Length of the incubation period associated with exposure and encroachment. Absorbed through the respiratory tract or gastrointestinal clinical manifestations of poisoning similar to anesthesia can occur in central nervous system and liver and kidney damage.
(2) the nervous system symptoms: dizziness, headache, fatigue, trance, gait, pankk Shan, brief unconsciousness or coma. Inhalation of high concentration may be due to inhibition of medulla oblongata and rapid coma, convulsions, and even sudden death.
(3) gastrointestinal symptoms: oral toxicity is more obvious. May have nausea, vomiting, loss of appetite, abdominal pain, diarrhea and jaundice, liver, liver tenderness signs of liver disease, liver function abnormalities, such as poisoning. Severe fulminant liver failure may occur. Incidence of liver damage symptoms more than di2-4 days.
(4) the kidney damage symptoms: there may be red cell urine, proteinuria, tubular urine. Appear severe oliguria, anuria, azotemia, acute renal failure.
(5) other: a few patients may have, arrhythmia, myocardial damage. Ventricular fibrillation and cause respiratory paralysis is death.
(6) the inhalation poisoning is often accompanied by eye and upper respiratory tract irritation. Sometimes can cause pulmonary edema.
2. chronic toxicity
Rare reports of chronic poisoning. Long-term repeated exposure to carbon tetrachloride can include dizziness, fatigue, insomnia, memory loss, loss of appetite, nausea, diarrhea, and abdominal pain. Liver, liver function abnormalities. Severe cases can develop into Portal cirrhosis. A few patients with retrobulbar neuritis, contraction of Visual field occurs, vision loss. In addition, foreign reports can cause hearing impairment, cochlear nerve and vestibular system dysfunction and aplastic anemia. Prolonged exposure of the skin, chapped due to dryness, scaling and skim.
Carbofuran
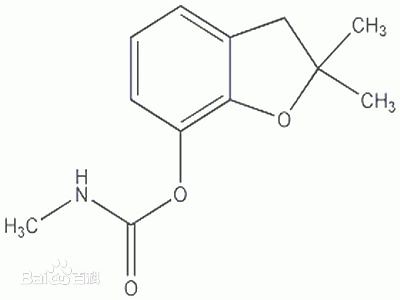
Carbofuran, chemical formula C12H15NO3 molecular weight 221.25, is a highly toxic pesticide, is a broad-spectrum insecticide, nematicide, with contact and stomach poison action. No irritation to eyes and skin. Carbofuran
It does not combine with cholinesterase reversibly, so toxicity is very high. Can be absorbed by plant roots, and transported to the various organs of plants, with leaf margins up. Carbofuran in soil residual life long, paddy field surface scatter short residual life. Usually applied to rice, cotton, tobacco, soybean and other crops on a variety of pest control can also be used specifically for use as a seed treatment.
Risk
Because birds can eat granular carbofuran pesticide deaths, United States environmental protection agency only allows this product to liquid form. In addition, from 2009, the United States environmental protection agency ordered a ban on any products containing residues of agricultural products intended for human consumption, whether locally produced or imported.
From October 1, 2015, the domestic requirement 199th Bulletin for the Department of agriculture to prohibit the use of carbofuran.
Methomyl
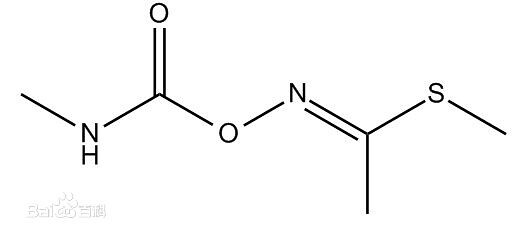
Also known as methomyl, methyl ethyl ketoxime Granville or ammonia-Cha-Wai. A broad spectrum of carbamate pesticides. White solid solubility in water is 58g/L, soluble in acetone, ethanol, methanol, isopropanol. Dosage form is a wettable powder. Mainly used in the control of rice stem borer, planthopper, prodenia litura and other pests. In 1966 by the United States at DuPont (DuPont), first featured as an insecticide, nematicide. Suitable for cotton, tobacco, fruit and vegetable fight pests such as aphids, moths, Tiger, is a good replacement of current prevention and treatment of drug-resistant cotton aphid species.
Methomyl is United States Environmental Protection Agency (EPA) classified as a first class highly toxic substances, human skin, lung, gastrointestinal, kidney, spleen, and blood-forming organs have varying degrees of toxicity, rat and rabbit animal studies found no teratogenic, mutagenic, and carcinogenic effects.
United States and United Kingdom provides operating environment the maximum allowable concentration in the air of 2.5mg/M3 (absorbed through the skin). Japan pesticide registration reservation standard given to 0.5mg/kg rice, vegetables, and fruit varieties for 1mg/kg.
Provided for in the standards promulgated by the Chinese Government: methomyl maximum pesticide residue limits are 3mg/kg, the EU maximum residue limit provided for 0.1mg/kg.
In 2014, according to the Ministry of agriculture Bulletin No. 2032, prohibition of methomyl in citrus trees, Apple tree, tea tree, and use on cruciferous vegetables
Risk
Methomyl is the World Health Organization (WHO) is defined as the highly toxic pesticide poisoning symptoms include dizziness, headache, severe drop in blood pressure can occur or unconsciousness, prolonged exposure to the pesticide will disrupt the human endocrine system.
Isoprocarb
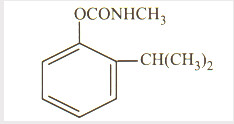
Isoprocarb also known as leafhoppers, destroyed Wei-bashing, is a contact and systemic action of insecticides, is a moderately toxic insecticides, insecticide with contact and stomach poison effects, quick, short residual life, used to control rice, cocoa, vegetables, sugar cane and other crops of rice planthopper and rice leafhopper and aphids, bugs, and so on. Planthopper predators, spiders safety but harmful to bees.
Risk
Health effects: symptoms include salivation, tearing, blurred vision, tremors, convulsions, delirium, coma, nausea, vomiting, diarrhea, abdominal pain, and finally died of respiratory failure.
Environmental hazard: a hazard to the environment, pollute the water and soil.
Explosion hazard: this product is flammable, highly toxic.
Cyano-naphthol
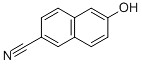
Name: 6-cyano-naphthol
English name: 6-Hydroxy-2-naphthonitrile
Molecular formula: C11H7NO
Molecular weight: 169.18
Water is a hazard, not allow undiluted or a lot of contact with ground water, water course or sewage system.
Reference sites
United States Environmental Protection Agency (U.S Environmental Protection Agency)
https://www3.epa.gov/airtoxics/hlthef/chlorobe.html
People's Republic of China Ministry of agriculture http://www.MOA.gov.CN/zwllm/tzgg/Gg/
Duke. The dictionary of chemistry. Beijing: chemical industry press. 2004. ISBN 7-5025-4409-7.
United States National Pesticide Information Center information http://extoxnet.orst.edu/pips/methomyl.htm
3.0 3.1 Carbofuran. Extension Toxicology Network. [April 19, 2014]
EPA Bans Carbofuran Pesticide Residues on Food. Environment News Service. [April 19, 2014]
No comments:
Post a Comment